Beta Thalassemia and related hemoglobinopathies (such as sickle cell disease), are usually characterized by high levels of HbF since this compensates for the lack of, or abnormal HbA that is expressed in vivo. Further insight on how the hemoglobin switch is controlled and regulated can become crucial in the treatment of hemoglobin disorders. The present treatment options currently include routine blood transfusions with the occasional complications attributed to iron overload in major organs. Human stem cell therapy and bone marrow transplantation are some other options that are very risky and not without complications.
Previous studies have shown that HbF levels differ in adults and that at least three loci independent of the β globin locus are responsible including XMN1, the HBS1L-MYB intergenic interval, and BCL11A. In another study conducted on an extended Maltese family, revealed that KLF1 also controls HbF levels in vivo. This study also suggested that other genes in conjunction with KLF1, play a part in activating the HbF switch upon birth.
To date, the complete mechanism of how globin gene switching works is still elusive. A total of 355 DNA samples were tested. The majority of the DNA samples were obtained from an existing collection of normal to borderline HbA2 and HbF cases, while other samples were collected along the course of the study. Data from anonymous and randomized cord blood DNA samples were also used and obtained from the Laboratory of Molecular Genetics, University of Malta. These were tested for the rs368698783 polymorphism under study and were used for reference purposes.
The SNP rs368698783 was amplified by PCR and checked by agarose gel electrophoresis using a 1.5% agarose gel. Restriction enzyme fragment length polymorphism analysis was then performed using Mnl1 restriction enzyme. Agarose gel electrophoresis was performed again in order to separate the Mnl1- incubated products according to size and thus determine the frequency of the mutation identified. Some samples were chosen at random and were genotyped by DNA sequencing.
Amongst the total study population (~355 individuals), the frequency of the mutant allele was 0.14 and close to 0.25 for the random newborn cord blood DNA analyzed in a previous study. The total study population was divided into 125 healthy adults and 193 into borderline HbF/HbA2
The mean fetal hemoglobin and HbA2 for the normal sub-population were 0.39± 0.034 and 2.68± 0.02 respectively. The mean levels in the borderline population were slightly higher with a mean of 0.6± 0.04 for fetal hemoglobin and 3.7±0.04 for HbA2
The effects of rs368698783 A allele on HbF levels examined in the total population and furthermore in the two sub populations of (i) normal healthy adults and (ii) adults with borderline levels of HbF/HbA2 showed that the mutant A allele of HBG1-rs368698783 exhibited a significantly elevated level of HbF (p < 0.05) compared with the wildtype G allele in each of the cohorts from Malta. Intriguingly no association was determined between this point mutation and HbA2 levels.
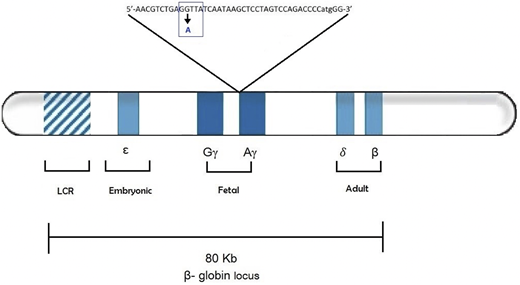
The data is suggestive that the variant HBG1-rs368698783 could act as a modifier associated with elevated levels of HbF in human adults. The SNP is located within the LYAR binding motif. The LYAR is a transcriptive factor which is said to silence the expression of fetal hemoglobin. thus, the G to A polymorphism reduces the DNA binding affinity increasing HbF levels invivo.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.